![¹H MAS NMR spectra for ethylene glycol [EG, C2H4(OH)2] embedded in the... | Download Scientific Diagram ¹H MAS NMR spectra for ethylene glycol [EG, C2H4(OH)2] embedded in the... | Download Scientific Diagram](https://www.researchgate.net/publication/324215697/figure/fig6/AS:960326023540764@1605970974824/H-MAS-NMR-spectra-for-ethylene-glycol-EG-C2H4OH2-embedded-in-the-beta-cages-of.gif)
¹H MAS NMR spectra for ethylene glycol [EG, C2H4(OH)2] embedded in the... | Download Scientific Diagram
![¹H MAS NMR spectrum for ethylene glycol [EG, C2H4(OH)2] adsorbed in NaX... | Download Scientific Diagram ¹H MAS NMR spectrum for ethylene glycol [EG, C2H4(OH)2] adsorbed in NaX... | Download Scientific Diagram](https://www.researchgate.net/publication/324215697/figure/fig5/AS:960326023516182@1605970974657/H-MAS-NMR-spectrum-for-ethylene-glycol-EG-C2H4OH2-adsorbed-in-NaX-zeolite-The_Q640.jpg)
¹H MAS NMR spectrum for ethylene glycol [EG, C2H4(OH)2] adsorbed in NaX... | Download Scientific Diagram
![An antifreeze solution is prepared from 222.6 g of ethylene glycol [C(2)H(4)(OH)(2)] and 200 g of water. Calculate the molality of the solution. If the density of the solution is 1.072g mL^(-1) An antifreeze solution is prepared from 222.6 g of ethylene glycol [C(2)H(4)(OH)(2)] and 200 g of water. Calculate the molality of the solution. If the density of the solution is 1.072g mL^(-1)](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/11880750_web.png)
An antifreeze solution is prepared from 222.6 g of ethylene glycol [C(2)H(4)(OH)(2)] and 200 g of water. Calculate the molality of the solution. If the density of the solution is 1.072g mL^(-1)
![Welcome to Chem Zipper.com......: How much ice will separate if a solution containing 25 g of ethylene glycol [C2H4(OH) 2] in 100g of water is cooled to 10°C? Kf(H2O) = 1.86 (25.05 Welcome to Chem Zipper.com......: How much ice will separate if a solution containing 25 g of ethylene glycol [C2H4(OH) 2] in 100g of water is cooled to 10°C? Kf(H2O) = 1.86 (25.05](https://lh3.googleusercontent.com/-l-d6OtTLXJA/XtYl5wlD-9I/AAAAAAAAHts/sC9TM2xK2iMGbrCcNXPwk7RHqGIui06GwCLcBGAsYHQ/w1200-h630-p-k-no-nu/1591092699610513-0.png)
Welcome to Chem Zipper.com......: How much ice will separate if a solution containing 25 g of ethylene glycol [C2H4(OH) 2] in 100g of water is cooled to 10°C? Kf(H2O) = 1.86 (25.05
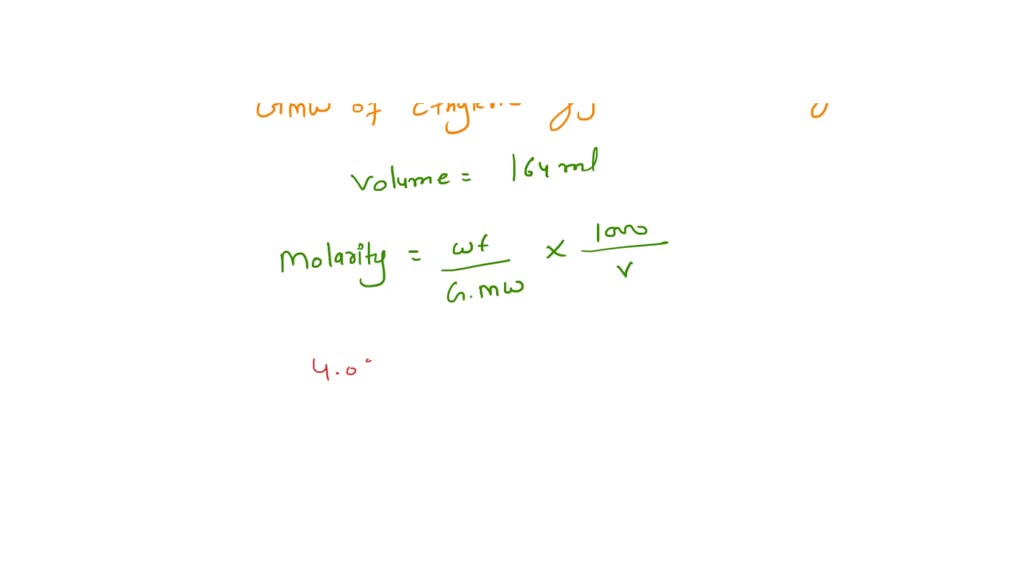
SOLVED: 1. Ethylene glycol is the primary component in antifreeze. How many grams of ethylene glycol (C2H4(OH)2) are present in 164 mL of a 4.024 M solution of antifreeze? 2. According to